Could This Psychedelic Drug Stock Be a Top Buy for 2026?

Amid a global mental health crisis, the demand for new and effective treatment options is on the rise, particularly in the realm of psychedelic-assisted therapies. Experts believe the psychedelic drug market will grow from $4.1 billion in 2025 to an impressive $7.8 billion by 2030, reflecting a steady compound annual growth rate (CAGR) of 13.7%.
As the space gains traction, one small biotech company has caught some investors' attention: Mind Medicine (MNMD), also known as MindMed for short. In its most recent earnings update, MindMed reported solid progress with enrollment across three pivotal Phase 3 trials for its lead candidate, MM120, a refined version of recreational drug lysergide d-tartrate (LSD) currently being studied for generalized anxiety disorder (GAD) and major depressive disorder (MDD).
Topline data from these trials is expected in 2026, setting the stage for a potential commercial launch by 2028. And analysts are also beginning to take notice. Oppenheimer, for example, recently expressed optimism based on what it believes could be “powerful” Phase 3 data next year. So, with clinical milestones approaching, would it be wise to buy the shares of this psychedelic drugmaker?
About Mind Medicine Stock
MindMed is a late-stage clinical biopharmaceutical company developing novel product candidates to treat brain health disorders. Its most advanced program, MM120, is currently in Phase 3 trials for GAD. Another key candidate, MM402, an MDMA-related compound, is in Phase 1 trials and being studied for its potential to address core symptoms of autism spectrum disorder (ASD).
With a pipeline focused on conditions that often lack effective long-term solutions, the company is aiming to bring a fresh approach to mental health treatment. Valued at roughly $747 million by market capitalization, shares of this psychedelic therapy-focused company are gaining traction as investors take note of its advancing clinical pipeline and growing role in mental health innovation.
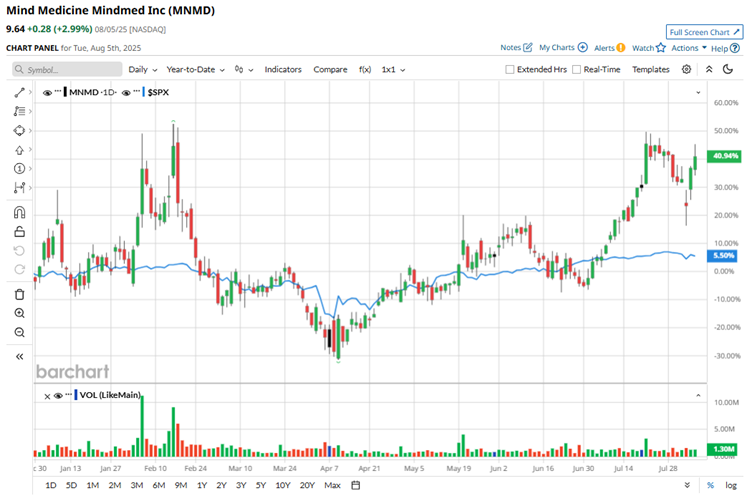
Over the past year, shares have risen 34%, and the rally has only intensified in 2025 with a year-to-date (YTD) gain of 41%. That easily tops the broader S&P 500 Index’s ($SPX) 22% gain over the past year and its 8% rise so far this year. Most notably, MindMed has surged 49% in just the last three months, highlighting growing investor interest.
A Look Inside Mind Medicine’s Q2 Earnings
MindMed’s fiscal 2025 second-quarter earnings, released on July 31, painted a mixed picture as the company deepens its push into late-stage development. The pre-revenue biotech firm posted a loss of $0.50 per share, widening from a loss of $0.26 a year ago and missing Wall Street’s forecast of a $0.38 loss per share. The shortfall was driven by a sharp increase in research and development expenses, which surged 103.5% year-over-year (YOY) to $29.8 million, primarily due to the advancement of the MM120 program.
While the company is investing heavily in advancing the MM120 program, one area of concern is the limited insight into the treatment’s long-term effectiveness. So far, Phase 2 data only tracks results up to 12 weeks, leaving questions about how durable the benefits may be over an extended period. That said, MindMed's financial position remains strong.
As of June 30, the company reported $237.9 million in cash, cash equivalents, and investments, enough to support operations into 2027 and beyond the first topline readout from its Phase 3 MM120 ODT trial. Meanwhile, clinical progress continues to move forward. MindMed remains on schedule with enrollment across three pivotal Phase 3 trials — Voyage, Panorama, and Emerge — which are focused on MM120 ODT for the treatment of GAD and MDD.
These studies are designed to assess the efficacy of MM120 ODT as a standalone treatment, without the need for psychotherapy. The company expects topline data from its Phase 3 Voyage trial in the first half of 2026, followed by Panorama and Emerge in the second half.
What Do Analysts Think About Mind Medicine Stock
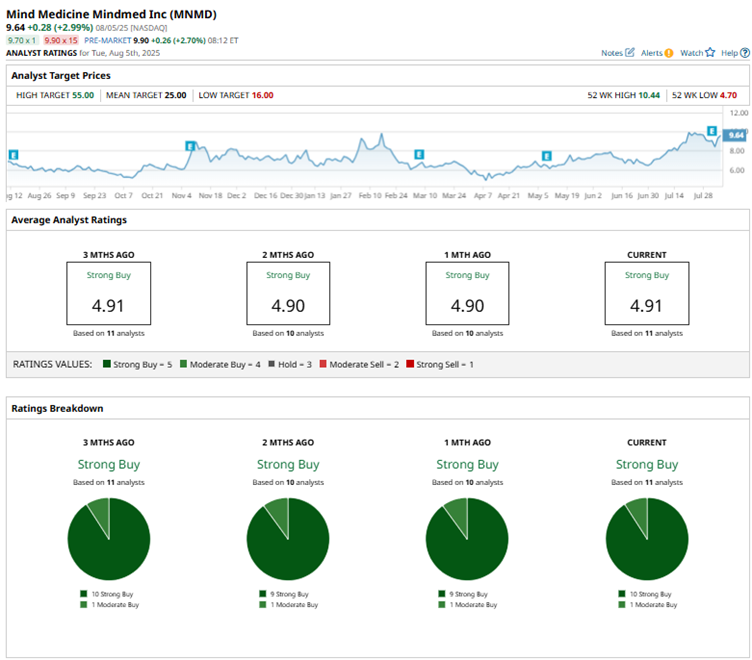
While investors' reaction to the company’s Q2 results was somewhat underwhelming, sentiment took a sharp positive turn on Aug. 4 when shares jumped nearly 11% following an upgrade from Oppenheimer. Analyst Jay Olson lifted the psychedelic drug developer to an “Outperform” rating and set a 12- to 18-month price target of $25, citing the promising potential of its lead candidate, MM120.
Olson highlighted MM120’s “unique clinical profile,” describing it as a pharmaceutical form of LSD that delivers a less intense but longer-lasting psychedelic experience compared to other approaches. The analyst also emphasized the drug’s potential in treating neuropsychiatric conditions like GAD and MDD.
Backing his optimism, Olson noted encouraging Phase 2 trial data for MM120 in GAD, which showed favorable remission rates as well as a favorable safety and tolerability profile over a 12-week period. In his view, MindMed remains undervalued and is strategically positioned ahead of its anticipated Phase 3 readout
Overall, MNMD stock has earned a resounding vote of confidence on Wall Street, carrying a consensus rating of “Strong Buy.” Of the 11 analysts covering the stock, a majority of 10 analysts recommend a "Strong Buy" while the remaining one gives a “Moderate Buy” rating. The average analyst price target of $25 indicates significant upside potential of 157% from the current price levels.
Final Thoughts
While MindMed’s journey comes with the usual biotech risks — especially given its pre-revenue status, rising R&D costs, and questions around the long-term durability of MM120’s effects — the company’s progress in late-stage trials, strong financial position, and growing confidence from Wall Street paint an encouraging picture. For investors comfortable with biotech volatility and looking to gain exposure to the emerging psychedelic therapy space, MindMed could be a promising buy ahead of its anticipated Phase 3 milestones in 2026.
On the date of publication, Anushka Mukherji did not have (either directly or indirectly) positions in any of the securities mentioned in this article. All information and data in this article is solely for informational purposes. For more information please view the Barchart Disclosure Policy here.